|
•
|
n is the principal quantum number.
|
|
•
|
l is the angular quantum number.
|
|
•
|
m is the magnetic quantum number.
|
|
•
|
|
•
|
|
•
|
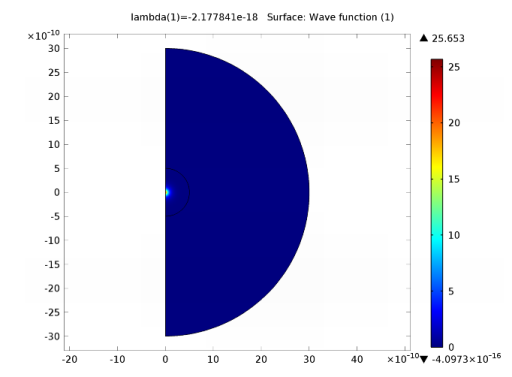
Figure 4-1: The wave function ψ for the first energy eigenvalue.
Comparing these numbers with the computed eigenvalues, you can see a 2-fold degeneracy for n = 2 and a 3-fold degeneracy for n = 3. This degeneracy corresponds to the following quantum triplets: (2,0,0) and (2,1,0); (3,0,0), (3,1,0), and (3,2,0). The computed values are separated due to the approximate numerical solution.
By refining the mesh and solving again, you can achieve more accurate results. The states with l = 0 correspond to spherically symmetric solutions, while states with l = 1 or 2 correspond to states with one or two radial node surfaces. The 0 energy level corresponds to the energy of a free electron no longer bounded to the nucleus. Energy levels closer to 0 correspond to excited states.
The wave function by itself has no direct physical interpretation. Another quantity to plot is ⏐Ψ⏐2, which is proportional to the probability density (unnormalized) function for the electron position after integration about the z-axis. The plot shows the unnormalized probability density function.